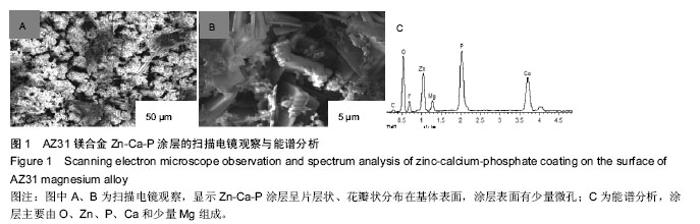
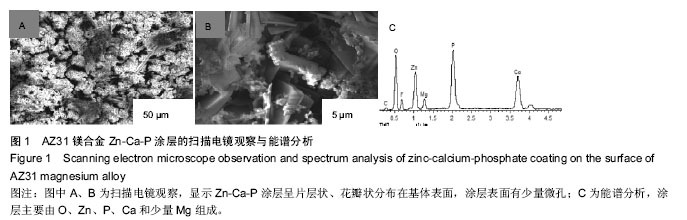
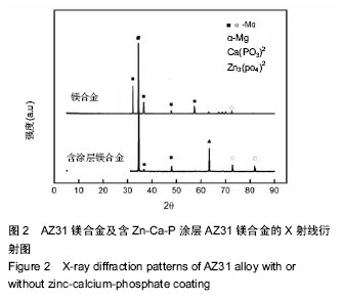
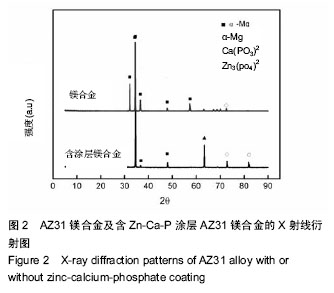
Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (25): 3720-3725.doi: 10.3969/j.issn.2095-4344.2016.25.011
Previous Articles Next Articles
Hemocompatibility of zinc-calcium-phosphate coating on the surface of AZ31 magnesium alloy in vitro
Zou Yu-hong1, Chen Yue1, Hu Min1, Wang Qing-zhao1, Zeng Rong-chang2
- 1School of Chemical and Environmental Engineering, 2School of Materials Science and Engineering, Shandong University of Science and Technology, Qingdao 266590, Shandong Province, China
-
Received:2016-04-08Online:2016-06-17Published:2016-06-17 -
Contact:Wang Qing-zhao, M.D., Professor, School of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, Shandong Province, China -
About author:Zou Yu-hong, Studying for doctorate, Associate professor, School of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, Shandong Province, China
CLC Number:
Cite this article
Zou Yu-hong, Chen Yue, Hu Min, Wang Qing-zhao, Zeng Rong-chang. Hemocompatibility of zinc-calcium-phosphate coating on the surface of AZ31 magnesium alloy in vitro[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(25): 3720-3725.
share this article
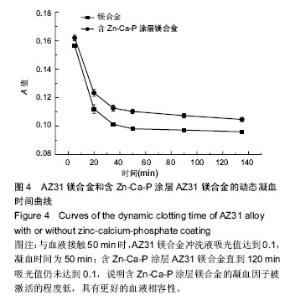
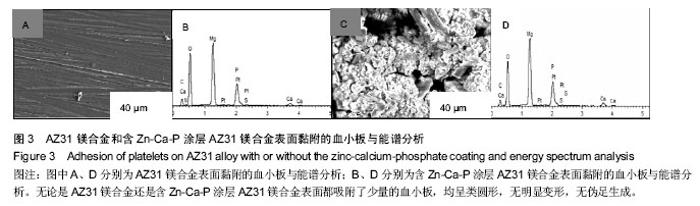
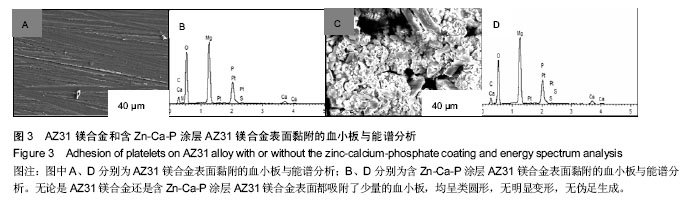
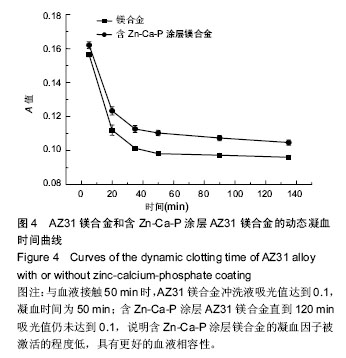
2.3 体外动态凝血时间实验结果 体外动态凝血时间可以间接检测内源性凝血因子被激活的程度,判断材料抗内源性凝血的能力[42],反映血液在材料表面的凝固趋势和凝血时间的长短。血液与材料接触连续增加的时间段内测定冲洗液的吸光度值越高,材料的体外动态凝血时间曲线越平缓,说明材料与血液接触后凝血因子被激活的程度越低,材料的血液相容性越好。为了比较不同材料的抗凝血性能,人为定义纵坐标吸光度值为 0.100 时的时间为材料的凝血时间。 AZ31镁合金比含Zn-Ca-P涂层AZ31镁合金的动态凝血曲线下降陡峭,与血液接触50 min时,AZ31镁合金冲洗液吸光值达到0.1,凝血时间为50 min;含Zn-Ca-P涂层AZ31镁合金直到120 min吸光值仍未达到0.1,见图4,说明含Zn-Ca-P涂层镁合金的凝血因子被激活的程度低,因此证明Zn-Ca-P涂层后的AZ31镁合金血液相容性更好。 2.4 溶血实验结果 溶血实验是评价材料血液相容性的常用指标,血液相容性好的医用材料应具有较低的溶血率(小于5%)[43-44]。实验中以测定545 nm波长处的吸光度值来计算溶血率。 AZ31镁合金的溶血率为(18.67±0.84)%,远远超出医用材料所规定的溶血要求;含Zn-Ca-P涂层AZ31镁合金的溶血率为(4.47±0.13)%,小于5%,满足对生物材料的溶血率要求。由此可知,经过Zn-Ca-P涂层后AZ31镁合金的溶血率降低明显,血液相容性较好。 对于AZ31镁合金处理前后溶血率的变化,分析机制可能为:未进行表面处理的AZ31镁合金和血液接触后,材料局部腐蚀,产生的镁离子,提高了血液的渗透压,导致红细胞膜的破裂,造成溶血。当对AZ31镁合金进行Zn-Ca-P涂层后,AZ31镁合金的抗腐蚀性能显著提高,当其和血液相接触时,材料的降解减慢,进入血液的镁离子减少,红细胞的破裂减少,溶血率下降。 "
| [1] 杨柯,谭丽丽,任伊宾,等.AZ31镁合金的生物降解行为研究[J].中国材料进展,2009, 28(2):26-30.[2] Zheng YF,Gu XN,Witte F.Biodegradable metals.Mater Sci Eng R.2014;77:1-34.[3] Zeng RC,Dietzel W,Witte F,et al.Progress and Challenge for Magnesium Alloys as Biomaterials.Adv Eng Mater.2008;10(8):B3-14. [4] Huehnerschulte TA,Angrisani N,Rittershaus D,et al.In Vivo Corrosion of Two Novel Magnesium Alloys ZEK100 and AX30 and Their Mechanical Suitability as Biodegradable Implants.Materials.2011;4:1144-1167. [5] Tapiero H,Tew KD.Trace elements in human physiology and pathology: zinc and metallothioneins.Biomed Pharmacother. 2003;57(9):399-411.[6] 俞耀庭,张兴栋.生物医用材料[M].天津:天津大学出版, 2000.[7] 颜廷亭.AZ31B镁合金的生物医用表面改性研究[D].南京南京理工大学,2010.[8] Zeng RC,Wang L,Zhang DF,et al.In vitro corrosion of Mg-6Zn-1Mn-4Sn-1.5Nd/0.5Y alloys.Front Mater Sci. 2014;8(3):230-243.[9] 王海涛.生物镁合金的生物相容性研究[D].郑州:郑州大学第一附属医院,2010.[10] Staigera MP,Pietaka AM.Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006; 27:1728-1734.[11] Zhou W,Zheng Y,Leeflang M,et al.Mechanical property, biocorrosion and in vitro biocompatibility evaluations of Mg-Li-(Al)-(RE) alloys for future cardiovascular stent application.Acta Biomaterialia.2013;9(10):8488-8498.[12] Zhu SJ,Liu Q,Qian YF,et al.Mechanical property and corrosion behavior in simulated body fluid of Mg-Zn-Y-Nd alloy for cardiovascular stent application. Front Mater Sci. 2014;8(3):256-263.[13] Stulikova I,Smola B.Mechanical properties and phase composition of potential biodegradable Mg-Zn-Mn- base alloys with addition of rare earth elements.Mater Charact. 2010;61:952-958.[14] Gu XN,Li N,Zhou WR,et al.Corrosion resistance and surface biocompatibility of a microarc oxidation coating on a Mg-Ca alloy.Acta Biomaterialia. 2011;7:1880- 1889.[15] Huang J,Dong P,Hao WC,et al.Biocompatibility of TiO2 and TiO2/heparin coatings on NiTi alloy.Appl Surf Sci. 2014;313:172-182.[16] 袁青领,阎钧,郑起,等.镁锌合金植入动物体内的分子生物相容性[J].中国组织工程研究,2013,17(16):2921-2926[17] Nene SS,Kashyap BP,Prabhu N,et al.Microstructure refinement and its effect on specific strength and bio-corrosion resistance in ultralight Mg–4Li–1Ca (LC41) alloy by hot rolling.J Alloys Compd. 2014;615:501-506.[18] Zeng RC,Wang L,Zhang DF,et al.In vitro corrosion of Mg-6Zn-1Mn-4Sn-1.5Nd/0.5Y alloys.Front Mater Sci. 2014;8(3):230-243.[19] Zeng RC,Qi WC,Song YW,et al.In vitro degradation of MAO/PLA coating on Mg-1.21Li-1.12Ca-1.0Y alloy. Front Mater Sci.2014;8(4):343-353.[20] Zeng R,Qi W,Zhang F,et al.In vitro corrosion of Mg–1. 21Li-1.12Ca-1Y alloy. Prog Nat Sci. 2014;24:492-499.[21] 吴璐,潘复生,杨明波,等.Mg-9Al-1Zn-(0-0.6)Sr镁合金铸态组织及晶粒细化研究[J].功能材料, 2014,45(2):2063-2067.[22] Funatania K.Emerging technology in surface modification of light metals.Surf Coat Technol. 2000; 133-134:264-272.[23] Jiang YF,Zhai CQ,Liu LF,et al.Zn-Ni alloy coatings pulse-plated on magnesium alloy.Surf Coat Technol. 2005;191:393-399. [24] Williams G,Grace R.Chloride-induced filiform corrosion of organic-coated magnesium.Electrochimica Acta. 2011;56(4):1894-1903.[25] Cui XF,Li Y,Li QF,et al.Influence of phytic acid concentration on performance of phytic acid conversion coatings on the AZ91D magnesium alloy.Mater Chem Phys.2008;111(2/3):503−507. [26] Tsn SN,Park TS,Lee MH.Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges.Prog Mater Sci. 2014;60:1-71.[27] Elsentriecy HH, Azumi K,Konno H.Improvement in stannate chemical conversion coatings on AZ91D magnesium alloy using the potentiostatic technique. Electrochimica Acta. 2007;53(2):1006-1012. [28] Zucchi F, Frignani A, Grassi V, et al.Stannate and permanganate conversion coatings on AZ31 magnesium alloy.Corros Sci.2007;49(12):4542-4552. [29] 颜廷亭,谭丽丽,熊党生,等.生物医用AZ31B镁合金表面稀土转化膜的制备及其性能[J].稀有金属材料与工程, 2009,38(5):918-923.[30] Lan W,Sun JC,Zhou AR,et al .Structures of zinc- manganese phosphate film and organic coating on magnesium alloys.Mater Sci Forum. 2009;610-613: 880-883.[31] 周婉秋,单大勇.镁合金的腐蚀行为与表面防护方法[J].材料保护,2002,35(7):1-3.[32] Kouisni L,Azzi M,Zertoubi M,et al.Phosphate coatings on magnesium alloy AM60 part 1: study of the formation and the growth of zinc phosphate films. Surf Coat Technol. 2004;185(1):58-67. [33] Kouisni L,Azzi M,Dalard F,et al.Phosphate coatings on magnesium alloy AM60 Part 2: Electrochemical behaviour in borate buffer solution.Surf Coat Technol. 2005;192:239-246.[34] Niu LY.Investigation on film formation mechanism, microstructure and performance of complex zinc phosphate coating of magnesium alloys. Changchun: Jilin University,2006:25.[35] Zeng RC,Sun XX,Song YW,et al.Influence of solution temperature on corrosion resistance of Zn-Ca phosphate conversion coating on biomedical Mg-Li-Ca alloys.Trans Nonferrous Met Soc China.2013;(23):3293-3299.[36] Zeng RC,Lan ZD.Influence of bath temperature of conversion treatment process on corrosion resistance of zinc calcium phosphate conversion film on AZ31 magnesium alloy. Chin J Nonferrous Metal.2010;20: 1461-1466.[37] Zeng RC,Zhang F,Lan ZD,et al.Corrosion resistance of calcium-modified zinc phosphate conversion coatings on magnesium-aluminium alloys.Corros Sci.2014;88: 452-459. [38] 曾荣昌,孔令鸿,许苏,等.医用Mg-Li-Ca合金表面Ca-P涂层腐蚀研究[J].重庆理工大学学报(自然科学版), 2015, 10(24):34-39. [39] 张明华,郑海燕.抗凝血生物材料的血液相容性[J].中国组织工程研究, 2013,17(8):1505-1512.[40] Chen B,Wang D.Preparation and mechanism of anticoagulatent biomedical polymer materials with blood compatibility.Zhong guo Zu zhi Gong cheng Yan jiu yu Lin chuang Kangfu.2011;15(29):5507-5510.[41] Grunkemeier JM,Tsai WB,Horbett TA.Co-adsorbed fibrinogen and von Willebrand factor augment platelet procoagulant activity and spreading.J Biomater Sci. 2001;12:1-20.[42] Wang Q,Tan LL,Xu WL,et al.Dynamic behaviors of a Ca-P coated AZ31B magnesium alloy during in vitro and in vivo degradations.Mater Sci Eng B. 2011;176: 1718-1726.[43] 陈旭琼,尹庆水,张余,等.镁合金材料的溶血率及影响因素[J].中国组织工程研究,2012,16(25):4632-4638.[44] Acker JP,Croteau IM,Yi QL.An analysis of the bias in red blood cell hemolysis measurement using several analytical approaches.Clinica Chimica Acta.2012;413: 1746-1752. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||